
|
Below we translate the front of the
Tomsk unit packaging (or "PDP" - principal display panel),
as well as the reverse - from Russian into English.
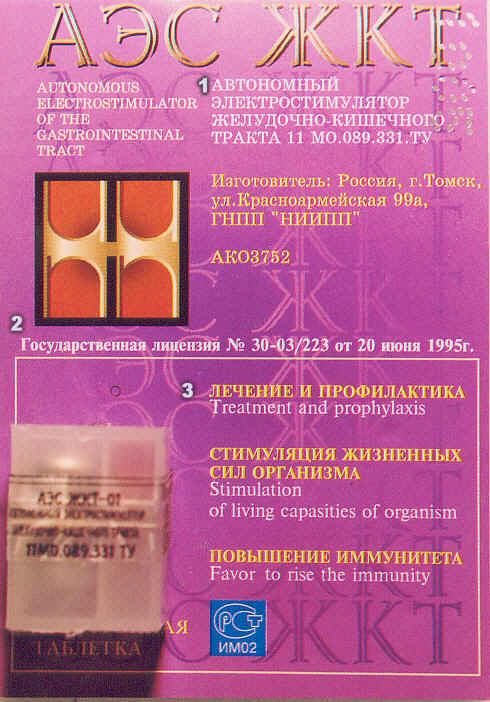
![]() he translation of the Tomsk units' packaging is rendered
below. Note that all three units have very similar language -
and virtually the same instructions. We group the language
into three areas -- numbered (1), (2), and (3).
he translation of the Tomsk units' packaging is rendered
below. Note that all three units have very similar language -
and virtually the same instructions. We group the language
into three areas -- numbered (1), (2), and (3).
![]() (1) ----
This descriptive is already rendered roughly in English
to the left of the Russian. The six cyrillic characters at
the top of the package stand for "Autonomous Electrostimulator
(AEC) of the Gastrointestinal Tract (GIT)" -- just as you
see at the top of this page and in off-white text to the
left of the cyrillic below it. The chromium and zinc
coated units add, "With
endogenous
electrophoresis (or
cataphoresis) of chromium (or zinc, if zinc-coated unit) ions (by Agafonnikov)."
(Apparently, the Russian scientist to whom credit is bestowed for
having invented the Tomsk units bears the last name, Agafonnikov).
(1) ----
This descriptive is already rendered roughly in English
to the left of the Russian. The six cyrillic characters at
the top of the package stand for "Autonomous Electrostimulator
(AEC) of the Gastrointestinal Tract (GIT)" -- just as you
see at the top of this page and in off-white text to the
left of the cyrillic below it. The chromium and zinc
coated units add, "With
endogenous
electrophoresis (or
cataphoresis) of chromium (or zinc, if zinc-coated unit) ions (by Agafonnikov)."
(Apparently, the Russian scientist to whom credit is bestowed for
having invented the Tomsk units bears the last name, Agafonnikov).
![]() The "11 MO.089.311.TY"
is the code for the "Technical Requirements" (the TY is
pronounced "TEE-OU" in Russian, not "TEE-WHY" -- the "OU"
as you say "yOU" without the letter "y" ... you see this
quite a bit and it presents the regulatory number under
which product is made. It reflects HOW the product is
made and allows it to be legally sold in Russia).
The "11 MO.089.311.TY"
is the code for the "Technical Requirements" (the TY is
pronounced "TEE-OU" in Russian, not "TEE-WHY" -- the "OU"
as you say "yOU" without the letter "y" ... you see this
quite a bit and it presents the regulatory number under
which product is made. It reflects HOW the product is
made and allows it to be legally sold in Russia).
![]() The yellow text
below it translates as: "Manufactured in: Russia - Tomsk"
(a major industrial and technical center in the Ural
Mountains of Russia - located in the southeast area
of Western Siberia - see
clickable
map of Russia and you'll find "TOM" region northeast
of Kazahkhsthan (sic)), "Krasnoarmeyskaya Street, 99a"
(the address of the manufacturer), and finally
the acronym for the manufacturing company itself:
"GNPP - 'NIIPP'" or "State Scientific and Production
Enterprise." There name implies both the scientific
research and production components of the enterprise. Below that
the model number assigned to the unit is given: "AKO3752."
The yellow text
below it translates as: "Manufactured in: Russia - Tomsk"
(a major industrial and technical center in the Ural
Mountains of Russia - located in the southeast area
of Western Siberia - see
clickable
map of Russia and you'll find "TOM" region northeast
of Kazahkhsthan (sic)), "Krasnoarmeyskaya Street, 99a"
(the address of the manufacturer), and finally
the acronym for the manufacturing company itself:
"GNPP - 'NIIPP'" or "State Scientific and Production
Enterprise." There name implies both the scientific
research and production components of the enterprise. Below that
the model number assigned to the unit is given: "AKO3752."
![]() (2) ---- The second section
is simple and straight-forward: "State License No. 30-03/223 of June 20, 1995."
This simply gives the number under which this device is legally registered with
the Russian Federation's Ministry of Health (the rough equivalent of the
"U.S. Food & Drug Administration" in the States) and the date this device
first received its license.
(2) ---- The second section
is simple and straight-forward: "State License No. 30-03/223 of June 20, 1995."
This simply gives the number under which this device is legally registered with
the Russian Federation's Ministry of Health (the rough equivalent of the
"U.S. Food & Drug Administration" in the States) and the date this device
first received its license.
![]() (3) ---- Here you see
English translations beneath the Russian. The last two translations
are awkward in English. Better translations would be "Stimulates Vitality"
in the second line; "Immunity Boosting", in the third.
(3) ---- Here you see
English translations beneath the Russian. The last two translations
are awkward in English. Better translations would be "Stimulates Vitality"
in the second line; "Immunity Boosting", in the third.
![]() At the very bottom left
in yellow Cyrillic letters (partially covered, below the plastic
casing), the tranlation would be 'energy of renewal,' or less
awkwardly, "Replenishes Your Energy."
At the very bottom left
in yellow Cyrillic letters (partially covered, below the plastic
casing), the tranlation would be 'energy of renewal,' or less
awkwardly, "Replenishes Your Energy."
![]() The black letters on the plastic
casing (hard to read, as it was difficult to focus) contains four
elements: a repeat of the description (Autonomus Electrostimulator...),
the comment "Quality Check Passed" (a quality assurance statement on
the left side panel),
a "best used before" followed by a date (a shelf-stability delimiter,
found on the right side panel), and finally, again on the face,
the "TY" regulatory number (see above).
The black letters on the plastic
casing (hard to read, as it was difficult to focus) contains four
elements: a repeat of the description (Autonomus Electrostimulator...),
the comment "Quality Check Passed" (a quality assurance statement on
the left side panel),
a "best used before" followed by a date (a shelf-stability delimiter,
found on the right side panel), and finally, again on the face,
the "TY" regulatory number (see above).
![]() Differences between models:
The language is nearly the same (you can see this for yourself in closeups
of the Basic
stainless steel model (our #1210), the
Chromium-plated model
(#1215), and the
Zinc-plated model
(#1220) ... also view composite
closeup).
Differences between models:
The language is nearly the same (you can see this for yourself in closeups
of the Basic
stainless steel model (our #1210), the
Chromium-plated model
(#1215), and the
Zinc-plated model
(#1220) ... also view composite
closeup).
![]() The only substantive difference is
the regulatory number through which the unit is sold with the blessings of the
Russian Federation's Ministry of Health.
The only substantive difference is
the regulatory number through which the unit is sold with the blessings of the
Russian Federation's Ministry of Health.

![]() he instructions on the back of the package read
as follows: "Approved for medical application for single use.
Non-Toxic. Apyrogenic (fever reducing)."
he instructions on the back of the package read
as follows: "Approved for medical application for single use.
Non-Toxic. Apyrogenic (fever reducing)."
![]() "For isolated electric
stimulation of stomach, duodenum, pancreatic duct, extra-liver tracts,
small intestines, colon during various diseases."
"For isolated electric
stimulation of stomach, duodenum, pancreatic duct, extra-liver tracts,
small intestines, colon during various diseases."
![]() "To check operation of the
electrostimulator, touch the caps of "AES GIT" with the indicator's
contacts; in one of the positions, the indicator should blink
once every 3 minutes."
"To check operation of the
electrostimulator, touch the caps of "AES GIT" with the indicator's
contacts; in one of the positions, the indicator should blink
once every 3 minutes."
![]() "YOUR BODY WILL HEAL
ITSELF WITH THE HELP OF AES GIT, which will awaken and synchronize
healthy powers and rhythms of your body, normalize its internal
organs' activity. The most important effect is the self-detoxification
and self-cleansing of the organism DUE TO THE RECOVERY OF NORMAL
FUNCTI0N OF THE ENTIRE GASTROINTESTINAL TRACT."
"YOUR BODY WILL HEAL
ITSELF WITH THE HELP OF AES GIT, which will awaken and synchronize
healthy powers and rhythms of your body, normalize its internal
organs' activity. The most important effect is the self-detoxification
and self-cleansing of the organism DUE TO THE RECOVERY OF NORMAL
FUNCTI0N OF THE ENTIRE GASTROINTESTINAL TRACT."